Abstract
Background: The use of electronic diaries (eDiaries) has been implemented as a promising strategy to improve the follow-up of diseases such as hemophilia, both for clinicians and for the patients themselves. In particular, standard treatment of hemophilia patients is mainly based on infusion of factor concentrates and is performed at home by patients or caregivers. This practice involves a thorough recording of infusions, bleedings, and other adverse events, which is usually carried out on paper and is deficient in most cases, making it very difficult to follow up and optimize patient treatment. Florio® HAEMO is a cell phone application (app) designed for hemophilia patients, where they can record bleeding and infusion details and share them with their physician in real time. The implementation of this app in the clinical routine of hemophilia patients promises to improve patient follow-up, enabling treatment at home in a more controlled and autonomous manner.
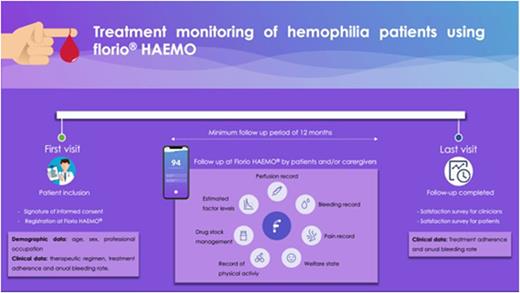
Methods: eSPLORHEM (Evaluation of Spanish experience of using florio HAEMO ediary) is a bispective, observational and multicenter study designed to evaluate the national experience on the use of florio HAEMO for treatment monitoring in hemophilia patients. Hemophilia A and B patients with factor levels <5%, with no other hemostasis problems and who are not participating in a phase 1-3 clinical trial will be included. The effect of the use of the app on adherence to treatment and the annual bleeding rate will be evaluated. Adherence to treatment will be calculated based on the prescribed treatment and concentrates collected at the pharmacy by the patient and ABR will be calculated by the number of bleedings for one year. Quality of life survey data will also be collected through the EQ-5D-5L questionnaire and at the end of the study a survey will be conducted with both patients and clinicians to assess the degree of satisfaction with the use of florio HAEMO. The quality of the documentation recorded in the app will be assessed by comparing the drug stock that the patient picks up at the pharmacy against the number of infusions and the stock recorded.
Results: To date, a total of 12 Spanish centers have confirmed their participation and it is estimated that a total of approximately 100 patients will be included. The protocol was approved by the competent authorities in January 2022 and is being implemented in all collaborating centers. The recruitment phase will be open until the end of August 2022, including a retrospective phase since May 2020 in order to include the experience of patients who have already started using the app. The follow-up period will end in August 2023, so that patients will complete a minimum follow-up period of 12 months. Final analysis report is expected to be available in November 2023.
Conclusion: The results of this study will provide objective evidence of the effect of the use of florio HAEMO, through clinical parameters such as treatment adherence and ABR, as well as the degree of patient and clinician satisfaction.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal